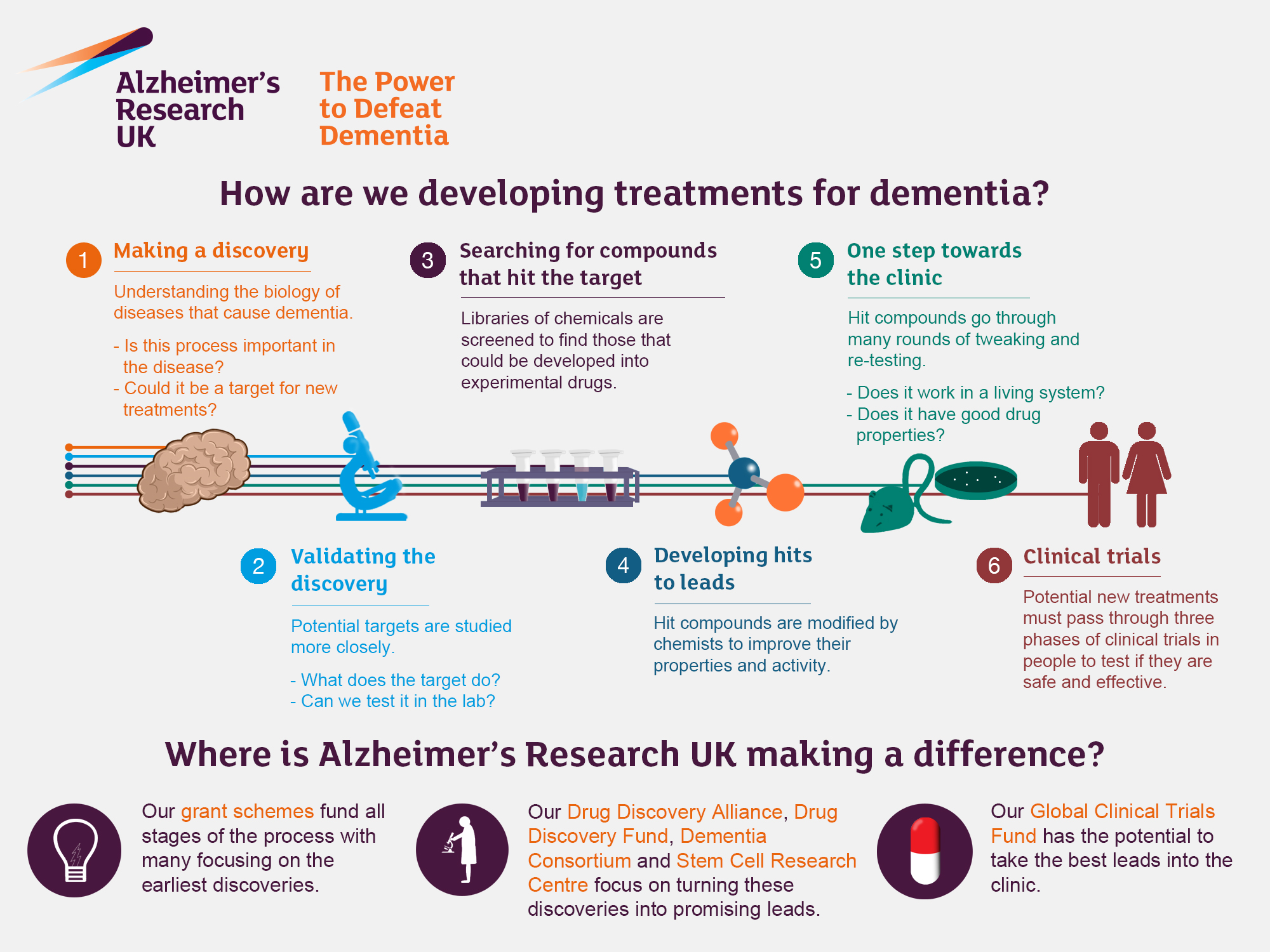
Alzheimer’s research is at the forefront of the fight against one of the most devastating neurodegenerative diseases impacting millions worldwide. Led by innovative scientists like Beth Stevens, this research uncovers the crucial role of microglial cells in the brain’s immune system, which are essential for maintaining neural health. These cells help to clear out cellular debris and manage synaptic connections, but when they malfunction, they can contribute to the progression of Alzheimer’s disease. With an aging population and a staggering rise in cases expected in the coming decades, breakthroughs in Alzheimer’s treatment have never been more critical. The ongoing studies in microglia and their implications for disease could pave the way for new therapies that significantly improve the lives of those affected.
Exploring advancements in Alzheimer’s disease studies reveals the critical insights into brain health and immune functions that could revolutionize our understanding and treatment of cognitive decline. Researchers are increasingly acknowledging the significance of glial cells and their influence on neuronal connectivity, ultimately shaping both normal brain development and the pathology of debilitating conditions such as dementia. As experts like Beth Stevens emphasize the myriad complexities of brain immune responses, the science shifts towards innovative strategies that could address this pressing health crisis. With the alarming prevalence of cognitive disorders rising, the emergence of novel therapeutic targets and biomarkers in the realm of neurobiology becomes essential. This multidimensional research landscape encourages optimism for effective Alzheimer’s interventions, aiming to transform future patient care.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells play a crucial role in the brain’s immune system, serving as the first line of defense against neurodegenerative diseases like Alzheimer’s. As researchers such as Beth Stevens delve deeper into the functions of these cells, it becomes evident that their seemingly simple task of maintaining brain health is far more complex. They are responsible for patrolling the brain, identifying harmful debris, and facilitating the pruning of synapses, which are essential for effective neuronal communication. However, when microglial activity becomes aberrant, it can lead to an increased risk of developing Alzheimer’s disease, highlighting the delicate balance they must maintain in brain health.
Recent studies led by Stevens’ lab have illustrated how dysfunction in microglial cells can exacerbate the pathology of Alzheimer’s. This transformation in understanding has paved the way for innovative Alzheimer’s treatment options that target these immune cells. By developing therapies that can restore the normal functions of microglia, researchers hope to mitigate the cognitive decline associated with Alzheimer’s. Stevens’ pioneering work not only contributes to the scientific community but also instills hope for the millions affected by this devastating illness.
The Role of Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning is an essential process in brain development and function, involving the removal of weaker synapses to enhance neural efficiency. At the forefront of Alzheimer’s research, this process is becoming increasingly critical as scientists like Beth Stevens uncover its implications on neurodegenerative diseases. When microglial cells excessively prune synapses, it can lead to detrimental effects on cognitive function and is believed to contribute to the onset of various neurodegenerative diseases, including Alzheimer’s. Understanding the molecular mechanisms behind this peculiar pruning may lead to effective interventions.
The insights gained from Stevens’ research highlight the importance of timely intervention in synaptic pruning to potentially reverse or prevent the cognitive impairments associated with Alzheimer’s. This research not only lays the groundwork for new treatment strategies but also presents an opportunity for early detection of Alzheimer’s disease through biomarkers related to microglial and synaptic activity. By focusing on these mechanisms, researchers aim to develop methods to monitor and potentially alter the disease progression, thereby improving the quality of life for patients.
Alzheimer’s Treatment: A Future Driven by Basic Science
The path to effective Alzheimer’s treatment is being charted through groundbreaking foundational research, emphasizing the significance of the inquiry-driven approach led by scientists like Beth Stevens. Despite the challenges of translating basic science into clinical applications, Stevens’ work illustrates the transformative potential of understanding neuroimmune interactions. Investing in such preliminary studies allows researchers to uncover the complexities of neurodegenerative diseases, identifying critical pathways that can lead to the development of promising therapies against Alzheimer’s.
Future Alzheimer’s treatments may leverage the discoveries made in the realm of microglial research, providing hope for millions at risk of neurodegenerative diseases. By enhancing the brain’s immune response and promoting healthy synaptic dynamics, we can open new avenues for intervention that could alter disease trajectories. The foresight and curiosity embodied in Stevens’ lab are essential drivers towards unraveling the intricacies of Alzheimer’s treatment, and harnessing these insights may one day lead to breakthroughs that change the course of treatment for this pervasive disease.
The Impact of Aging on Alzheimer’s Disease
Aging is a significant risk factor for developing Alzheimer’s disease, and understanding this relationship is crucial for effective treatment strategies. As the population continues to age, the prevalence of Alzheimer’s is expected to double, placing unprecedented pressure on healthcare systems. Research efforts directed at deciphering the cellular and molecular changes occurring in the brain with age can provide insights into not only the nature of Alzheimer’s but also potential preventative measures. Stevens’ research highlights how age-related changes in microglial function might contribute to increased vulnerability to neurodegenerative diseases.
Moreover, the age-related decline in the brain’s immune response plays a pivotal role in the disease’s progression. Stevens emphasizes the importance of recognizing how microglial cells adapt or fail to adapt with age, leading to an imbalance that can prompt the onset of Alzheimer’s. By focusing on the aging brain’s immune dynamics, scientists can cultivate an understanding that informs both treatment and prevention efforts, ultimately aiming to enhance brain health as individuals age.
Beth Stevens: A Catalyst for Change in Alzheimer’s Research
Beth Stevens has emerged as a pioneering figure in the field of Alzheimer’s research, primarily due to her insights into microglial functions. Her assertion that ‘the science has driven me’ epitomizes the dedication required to navigate the complex landscape of neurodegenerative diseases. By framing the immune response of the brain as a potential avenue for developing Alzheimer’s treatments, Stevens has significantly shifted the narrative around neurobiology and disease management. This reorientation is crucial for fostering innovative solutions in the fight against Alzheimer’s.
Moreover, Stevens’ recognition with the MacArthur “genius” grant reflects her contributions to both basic and translational science. Her work not only sheds light on the fundamental biological processes influencing Alzheimer’s but also encourages a collaborative approach where curiosity-driven research can lead to health advancements. By continuously advocating for robust funding and support for basic science, Stevens exemplifies how dedication to research can cultivate new pathways for understanding and combating Alzheimer’s disease.
Innovative Approaches to Alzheimer’s Diagnostics
Detecting Alzheimer’s disease at an early stage is paramount for effective intervention, and research led by scientists like Beth Stevens is spearheading innovative approaches to diagnostics. Identifying unique biomarkers associated with microglial activity could enable practitioners to diagnose Alzheimer’s much earlier than current methods allow. This shift in focus from symptomatic treatment to proactive diagnosis has the potential to revolutionize the landscape of Alzheimer’s care, providing patients with better outcomes through early intervention.
Recent findings suggest that changes in the function of microglial cells may serve as an indicator of Alzheimer’s progression. By monitoring these changes, researchers can develop non-invasive diagnostic tests that help identify individuals at risk. Stevens’ ongoing commitment to elucidating the relationship between brain immune responses and disease biomarkers reflects a broader push toward refining diagnostic capabilities, ensuring that timely and effective treatment options are available for those diagnosed with Alzheimer’s.
The Economic Burden of Alzheimer’s Disease
Alzheimer’s disease poses not only a health challenge but also a significant economic burden on families and society as a whole. With projections indicating that cases may nearly triple by 2050, the financial implications are staggering, with costs potentially rising to $1 trillion annually. Researchers like Beth Stevens are acutely aware of these ramifications, and her work emphasizes the urgent need for effective Alzheimer’s treatment and preventative strategies to alleviate this burden.
Investing in research that focuses on the underlying mechanisms of Alzheimer’s can yield substantial dividends by potentially reducing the overall prevalence and associated costs of the disease. Understanding the role of microglial cells in disease progression opens doors to developing targeted interventions that could slow down or even prevent the onset of Alzheimer’s. By tackling the fundamental issues of neurodegenerative diseases, we can reshape the economic landscape and preserve both the well-being of patients and financial resources.
The Future of Alzheimer’s Research: Collaborative Efforts
The complexity of Alzheimer’s disease necessitates a collaborative approach, bringing together scientists, clinicians, and policymakers to address the challenges it presents. Beth Stevens emphasizes the importance of interdisciplinary collaboration, highlighting how collective efforts can expedite advancements in Alzheimer’s research. By pooling expertise from various fields, researchers can tackle complex problems more effectively, ultimately leading to innovative treatment solutions for neurodegenerative diseases.
Furthermore, fostering partnerships between academic institutions and healthcare organizations enhances the translation of research findings into clinical applications. As Stevens advocates for continued funding and support for research initiatives, the spirit of collaboration becomes an essential component driving discovery and application in Alzheimer’s treatment. Together, the scientific community can harness the power of collaboration to revolutionize the future of Alzheimer’s research, ensuring that progress is made at every level.
Frequently Asked Questions
How do microglial cells relate to Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research because they serve as the brain’s immune system. They actively patrol for signs of illness or injury, clear out damaged cells, and regulate synaptic pruning. Aberrant functioning of these cells has been linked to the progression of Alzheimer’s disease and other neurodegenerative disorders.
What role does Beth Stevens play in Alzheimer’s research?
Beth Stevens is a prominent neuroscientist in Alzheimer’s research known for her groundbreaking work on microglial cells. Her research at Boston Children’s Hospital focuses on how these immune cells affect brain health and contribute to neurodegenerative diseases, providing insights that could lead to innovative treatments for Alzheimer’s.
What impact does Stevens’ research have on Alzheimer’s treatment?
Stevens’ research on microglial cells paves the way for new treatments for Alzheimer’s disease by uncovering how these cells can mistakenly prune healthy neurons. Understanding this process can lead to the development of therapies aimed at preventing or reversing the effects of Alzheimer’s, ultimately improving patient outcomes.
Can you explain the connection between microglial function and Alzheimer’s disease?
Understanding the microglial function is central to Alzheimer’s research as these cells help maintain brain health by removing waste and modulating synaptic connections. However, when microglia malfunction, their pruning processes can exacerbate neurodegenerative diseases like Alzheimer’s, emphasizing the need for targeted research in this area.
Why is early detection important in Alzheimer’s research?
Early detection is vital in Alzheimer’s research because it allows for timely intervention that could slow the disease’s progression. Research by Beth Stevens and her team aims to identify new biomarkers linked to microglial cell activity, which could facilitate earlier diagnoses and potentially improve treatment outcomes for affected individuals.
What future directions does Stevens’ research suggest for Alzheimer’s disease?
The future directions of Stevens’ research suggest a focus on developing therapeutics targeting microglial cells to correct their dysfunctional pruning in Alzheimer’s disease. Additionally, her work emphasizes the importance of continuous innovation in basic science, which can lead to groundbreaking findings that inform Alzheimer’s treatment strategies.
How does the brain’s immune system influence neurodegenerative diseases like Alzheimer’s?
The brain’s immune system, primarily through microglial cells, plays a critical role in neurodegenerative diseases like Alzheimer’s. These cells are responsible for maintaining neuronal health, but when dysregulated, they can contribute to the pathogenesis of Alzheimer’s by promoting inflammation and damaging neural circuitry.
What advancements in Alzheimer’s treatment have stemmed from studies of microglia?
Advancements in Alzheimer’s treatment arising from studies of microglia include the development of potential drugs aimed at modulating microglial activity to restore proper synaptic pruning. Stevens’ research has set the stage for creating therapies that may slow the onset or progression of Alzheimer’s in patients.
| Key Points | |
|---|---|
| Researcher | Beth Stevens, Associate Professor of Neurology, Harvard Medical School |
| Focus of Research | Microglial cells and their role in Alzheimer’s disease and other neurodegenerative disorders |
| Research Findings | Aberrant pruning by microglia can contribute to Alzheimer’s and Huntington’s diseases |
| Potential Impact | Development of new treatments and biomarkers for Alzheimer’s affecting 7 million Americans |
| Funding Sources | Primarily funded by federal agencies like the National Institutes of Health |
| Quote | “We would never have been able to move forward without the basic science and curiosity-driven science from the beginning.” – Beth Stevens |
Summary
Alzheimer’s research is crucial in understanding and combatting the effects of this devastating disease. Beth Stevens’ innovative studies on microglial cells reveal how these brain immune cells can influence neurodegeneration, particularly in Alzheimer’s and Huntington’s diseases. The significant advancements in biomarkers and treatment options stemming from her research hold promise for improving the lives of millions affected by Alzheimer’s. Continued support for foundational research is essential, as it underpins the discovery of effective therapies and enhances our understanding of complex neurological conditions.